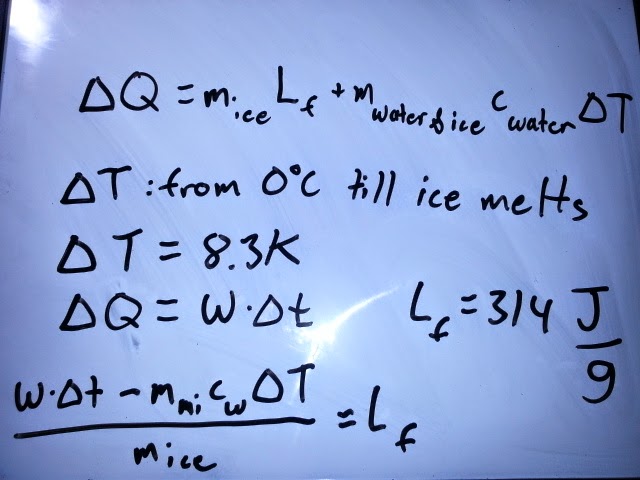At the start of class we were asked about what would a ring of metal do if it is heated.
 |
| White board work. We said that the metal ring we grow outward. |
Later Prof. Mason asked about would would the "Clinton" do if the brass side was heated or cooled.
 |
| White board work. If the brass is heated it will expand, if the brass is cooled it will contract. |
 |
| White board work. Algebra. |
Prof. Mason then did some real life demos. Video below.
 |
| Heating the brass side. Swings left like Clinton... |
 |
| Cooling the brass side. Swings right. |
Afterwards there was an experiment set up of a metal rod that would expand in thanks
to water steamed on to it. Video below.
 |
| The steam will make the metal rod grow and we would know how much by the change in theta. |
We did some calculations to find how much the metal expanded.
 |
| White board work. We think the metal rod is made of Aluminum. |
Afterwards we graphed our prediction of the heating curve 0 C of ice cold water.
 |
| Our prediction. The water would heat linear in till 100 C and then level out once it starts boiling. |
|
|
|
Apparatus used: Water heater to heat water, logger pro to get data, thermostat to read temperature, and Styrofoam cup to hold water.
 |
| Picture of apparatus'. |
 |
| Picture of experiment. |
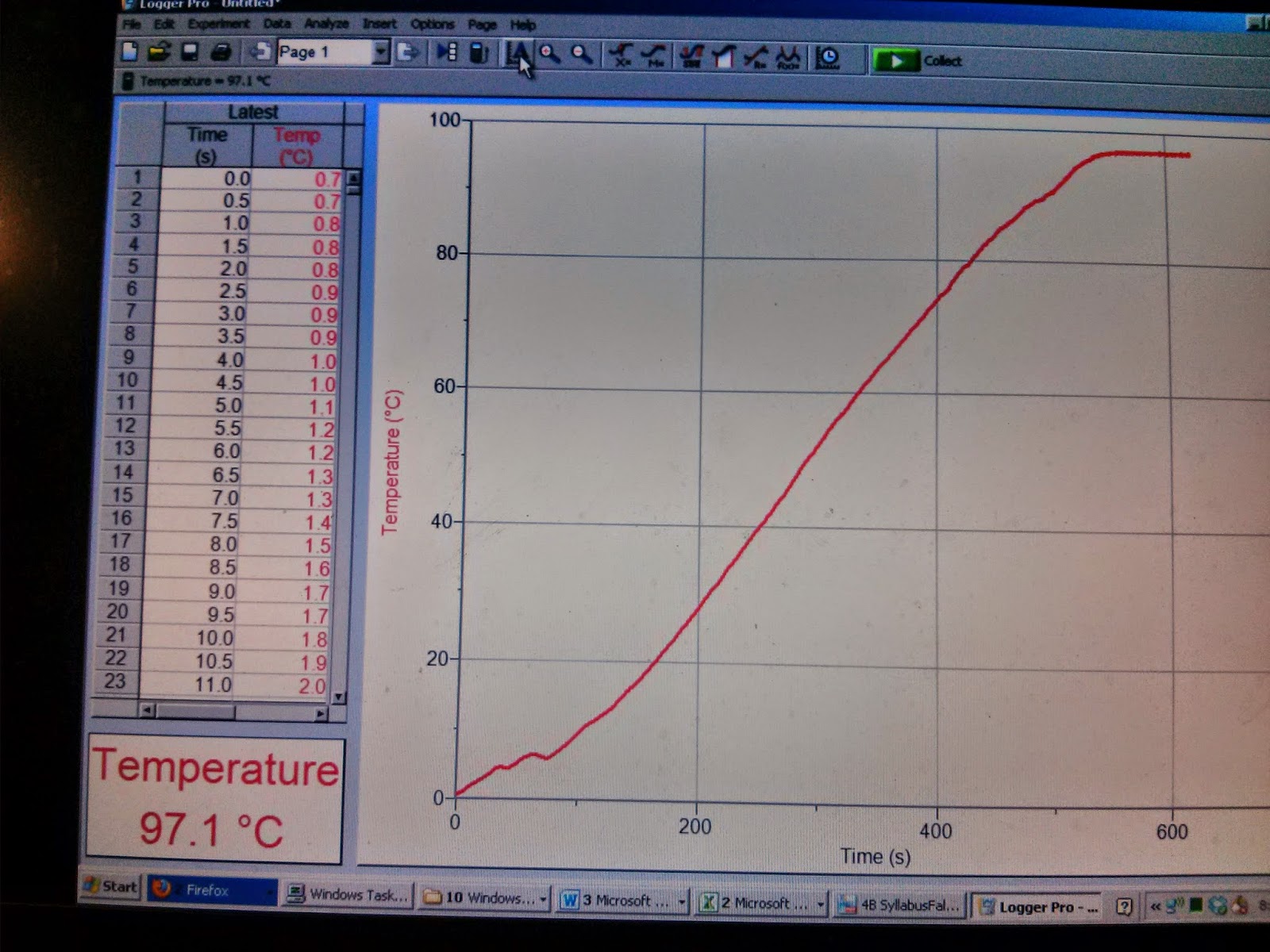 |
| The graph of the experiment of heating 0 C water. It matched our prediction pretty close. |
Afterwards we had to complete a lab that would help us understand latent heat of fusion and latent heat of vaporization. The lab required us to set up initial steps before starting the lab and find out how we would quantify the energies of latent fusion and latent vaporization.
 |
| White board work of our steps and data of experiment. |
 |
| White board of our collected data and possible error. |
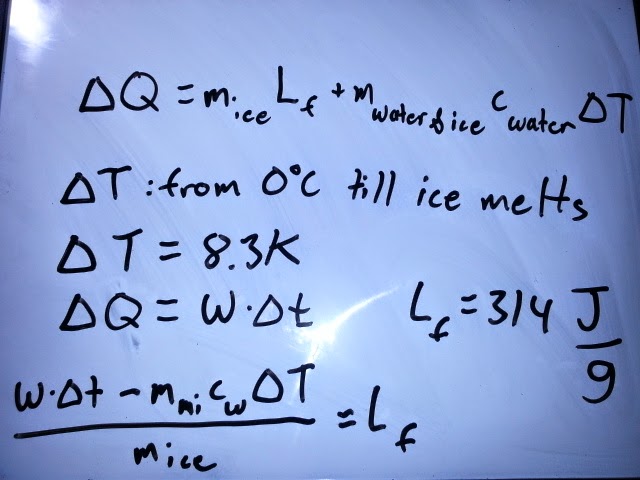 |
| White board work of latent heat of fusion. |
 |
| Propagation of error in specific heat. |
 |
| White board work of our steps and calculations. Experiment was redone for better results. |
 |
| White board work of our steps and calculations. This time to find latent heat of vaporization. |
Graph of experimental data taken by logger pro below.
 |
Graph of temperature vs. heat. There graph is linear and fitted.
|

Lastly we work out an example problem.
 |
| White board work of example problem. |
Summary of the day two.
We learned that each type of metal grows and contracts depending on the metal type. We had couple examples of this, one being the metal ring, metal rod, the "Clinton", Each one behaved differently when heated and cooled.
We learned about latent heat of fusion and latent heat of vaporization. We solidified what Prof. Mason discussed by doing a lab of mixing and recording data of ice cold water being melted and warmed and then heating to the boiling point.
Did some example problems of latent heat. Sometimes there is not enough energy in a system to merit a phase change. This was shown by the example done in class.